Triastek's 3D Printed Gastric Retention Product T22 Receives FDA Clearance of IND Application
- Triastek's 3D printed gastric retention product T22 received IND clearance from the FDA, becoming the world's first 3D printed gastric retention product.
- Currently, Triastek has four 3D printed products, T19, T20, T21, and T22, that have obtained IND clearance from the FDA, ranking Triastek first in the global 3D printed field for pharmaceutical clinical development.
NANJING, CHINA - Media OutReach Newswire - 1 February 2024 - On January 27, Triastek announced that the U.S. Food and Drug Administration (FDA) granted clearance to proceed for the Investigational New Drug (IND) of the company's 3D-printed product T22, making it the first 3D printed gastric retention product to receive this designation. Triastek is preparing to initiate clinical studies with T22 to fast-track product development.
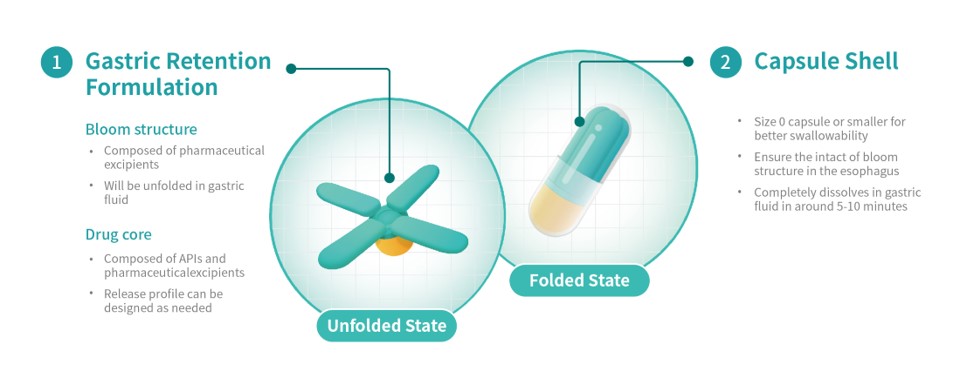
Triastek's T22 product is a 505(b)(2) product for the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). T22 is produced using Triastek's innovative Melt Extrusion Deposition plus Micro-Injection Molding (MED&MIM) process and utilizes its 3D Microstructure for Gastric Retention (3DμS®-GR) delivery technology platform. Compared with the current three times a day dosing of the originator product, T22 reduces the dosing frequency to once a day, simplifying the dosing regimen and improving medication adherence.
Dr. Senping Cheng, founder and CEO of Triastek, said: "Based on our proprietary 3D Microstructure for Gastric Retention delivery technology platform, the two products we developed, T20G and T22, have received IND clearance to proceed from regulatory agencies in China and the United States this year, marking the successful first step for Triastek's this innovative delivery technology platform proceeding through regulatory review process.
In 2021, Triastek and Sperogenix Therapeutics reached a co-development agreement regarding development and commercialization of T22 in East Asia to demonstrate the clinical application value of the 3D Microstructure Gastric Retention delivery technology. Based on the progress of T22, companies from several countries and regions have expressed interest in potential collaborations for product development utilizing this drug delivery technology platform."
Triastek has completed development of the T22 gastric retention formulation, achieved positive results in terms of in vitro expansion time, mechanical strength and dissolution behavior, and completed PK studies of the T22 gastric retention prototype in beagle dogs. Pharmacokinetic studies demonstrated that once daily dosing of the same total daily dose of the T22 gastric retention prototype gave comparable PK parameters, as TID dosing of the originator product.
With the FDA clearance to proceed with T22, this brings a total of four Triastek 3D-printed drug products, T19, T20, T21 and T22, to the clinical development stage, ranking first in the global 3D-printed drug field in terms of development product count. With the rapid advancement of the T-series pipeline and continuing validation of the clinical value of 3D printing drug technology, Triastek continues to develop new technologies and products for the global market. Currently our two key business models include "Product License-out Partnership" and "Technology Platform Partnership".
About Triastek's 3D Microstructure for Gastric Retention Delivery Technology Platform
A PCT application has been submitted for the 3D Microstructure for Gastric Retention delivery technology and its unique Bloom Structure design developed by Triastek. Upon oral administration, the gastric retention prototype expands to a size larger than the diameter of the pylorus, prolonging gastric retention time. During the gastric retention period, the prototype releases APIs according to a predetermined programmed drug release behavior. While simplifying the dosage regimen, reducing the medication burden, and improving the patient's long-term medication adherence, it can also improve drug absorption and oral bioavailability resulting in improved patient outcomes.
Hashtag: #Triastek#drug#3D-Printed
The issuer is solely responsible for the content of this announcement.

















